GET PROPEL
Stay Informed






The PROPEL Mini You Know.
NOW With a Straight Delivery System

| SINUS INDICATION |
DELIVERY ANGLE |
DELIVERY SYSTEM DIAMETER |
CATALOG # | ||
|---|---|---|---|---|---|
 |
Straight Delivery System |
Ethmoid sinus | Straight | 3.5 mm | 60044 |
 |
Curved Delivery System |
Ethmoid sinus Frontal sinus ostia |
Curved (S-shaped) |
4.0 mm | 60011 |
 |
 |
|
| Straight Delivery System |
Curved Delivery System |
|
| SINUS INDICATION |
Ethmoid sinus | Ethmoid sinus Frontal sinus ostia |
| DELIVERY ANGLE |
Straight | Curved (S-shaped) |
| DELIVERY SYSTEM DIAMETER |
3.5 mm | 4.0 mm |
| CATALOG # | 60044 | 60011 |
For your patients undergoing primary or revision ethmoid sinus surgery, who need a smaller alternative to PROPEL, with or without nasal polyps:
In the operating room
In the office
Watch Dr. Raithatha discuss the rationale for using PROPEL Mini deployed with the straight delivery system, in a 71-year-old male suffering from ethmoid sinus disease
HISTORY AND PRESENTING SYMPTOMS*
Multi-year history of chronic nasal congestion due to nasal polyps
Recent treatments include 3 rounds of prednisone over the past 3 months, steroid and antihistamine nasal sprays, and saline rinses
CT of the sinuses showed bilateral chronic pansinusitis with most involvement of his bilateral maxillary/ethmoid sinuses
Underwent septoplasty and bilateral endoscopic sinus surgery
Next steps: PROPEL Mini placement with the Straight Delivery System
*This case is intended for general medical education purposes only and is not a substitute for independent clinical medical judgment.
Response to treatment may vary from patient to patient.
≥18 years of age1,2
With and without polyps3
Undergoing primary or revision ethmoid sinus surgery3




PROPEL reduced the need for postoperative interventions vs a non-drug implant, at 30 days following ethmoid sinus surgery (32.8% vs 50.8%; N=143)*†
In a subgroup analysis, both patients with and without polyps experienced significant relative reductions in the need for postoperative interventions vs a non-drug implant


With polyps (n=91):
36% reduction
(32.5% vs 50.6%)


Without polyps (n=52):
35% reduction
(33.3% vs 51.1%)
*Postoperative interventions was a composite endpoint that included surgical intervention required to separate an adhesion and/or oral steroid intervention to resolve recurrent ethmoid sinus inflammation, edema, and/or polyp recurrence.
†Judged by an independent panel.
The benefits of PROPEL in reducing the need for postoperative interventions vs a non-drug implant following ethmoid sinus surgery were supported by reductions in oral steroids, polyposis, adhesions/scarring, and middle turbinate lateralization3
MT, middle turbinate.
*Judged by independent panel.
†Judged by on-site clinical investigators.
Study Design: Data presented here represent a meta-analysis of two prospective, randomized, double-blinded multicenter studies (Pilot and ADVANCE II) that enrolled a total of 143 patients. The studies evaluated outcomes of ethmoid sinus surgery with PROPEL placement compared to a non-drug implant, both with standard postoperative care. The studies used an intra-patient control design to evaluate clinical outcomes.3

Surgery + PROPEL significantly reduced patient-reported symptoms through
RSDI, Rhinosinusitis Disability Index; SNOT-22, Sino-Nasal Outcome Test-22.
*SNOT-22 evaluates rhinologic, extra-nasal rhinologic, ear/facial, psychological dysfunction and sleep dysfunction symptoms.4 RSDI evaluates physical, functional, and emotional symptoms.5
Learn more about the benefits of PROPEL after ethmoid sinus surgery
For significant improvements to patient outcomes following sinus surgery, add PROPEL to your action plan for the ethmoid sinus
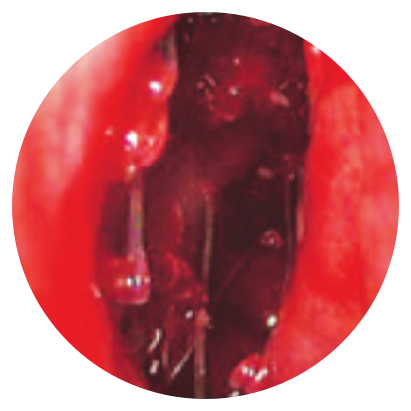
Day 0

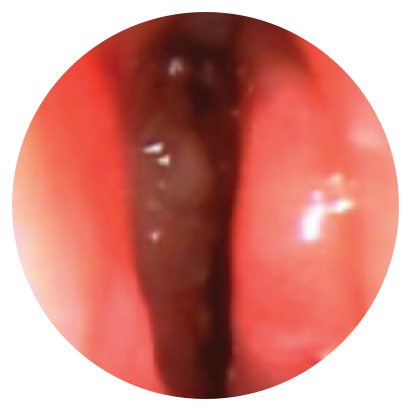
Day 30
Day 0
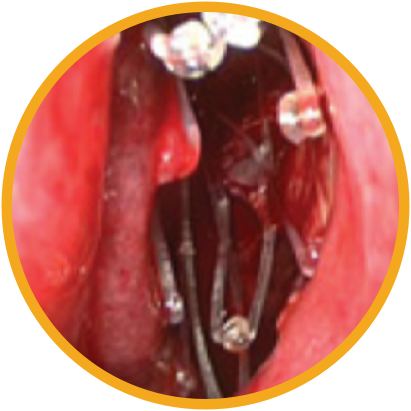

Day 30
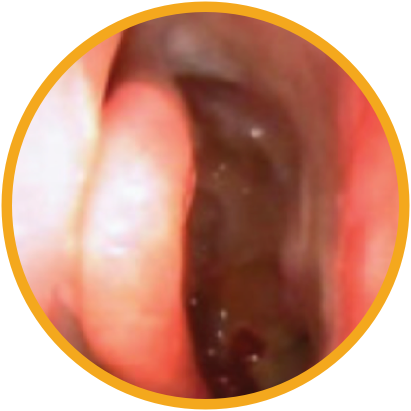
*Representative outcomes in bilateral sinuses of a single patient from a PROPEL clinical study. Individual results may vary.
Optimal treatment following ethmoid sinus surgery with a full arsenal of options including PROPEL and PROPEL Mini can help improve patient outcomes
The safety profile of PROPEL was established across three prospective clinical trials including 205 patients.1 The most common adverse reactions observed in ≥2% were sinusitis, headache, epistaxis, and bronchitis.
GET PROPEL
Stay Informed
This site is intended for healthcare professionals in the United States