GET PROPEL
Stay Informed


Provides your sinus surgery patients with individualized treatment options1-3
ESS, endoscopic sinus surgery.
Cutting-edge technology that opens the sinus cavity and delivers mometasone furoate1-3
Delivers the power of robust, clinically proven benefits1-3
PROPEL
PROPEL Mini
PROPEL Contour
Proven success in CRS patients1-6
Efficacious regardless of disease severity4
PROPEL demonstrated efficacy across multiple patient types:
Intersect ENT partnership and collaboration
CRS, chronic rhinosinusitis.


Watch Dr. Matheny discuss the postoperative use of PROPEL in the office, following a bilateral ethmoidectomy in an ambulatory surgery center
HISTORY AND PRESENTING SYMPTOMS*
Long history of CRS without nasal polyps and allergic rhinitis
Maximally managed with daily allergy medication, immunotherapy, antibiotics (5-6 times per year), and oral steroids (3-4 times per year)
Continued to suffer from nasal obstruction, facial pain and pressure, periorbital headache, changes to taste and smell, and nasal drainage
Underwent total bilateral ethmoidectomy with concurrent balloon catheter dilation of the frontal and maxillary sinus outflow tracts, in an ambulatory surgery setting
In this ambulatory surgery setting, the patient did not have access to PROPEL
Next steps: Postoperative PROPEL placement in the office
*This case is intended for general medical education purposes only and is not a substitute for independent clinical medical judgment.
Response to treatment may vary from patient to patient.
Improve sinus surgery outcomes for your patients with CRS by offering PROPEL implants in your in-office practice through a two-pronged approach: in-office sinus procedures or postoperatively.


PROPEL
IN-OFFICE
IMPLANT
OPTIONS
1
IN-OFFICE SINUS PROCEDURES
Implants following in-office ESS such as balloon sinus dilation
2
POSTOPERATIVE UTILIZATION
Implants during the postoperative healing period after sinus surgery
CLINICAL APPLICATION
COVERAGE
VALUE TO YOUR PRACTICE
Actor portrayal with simulated endoscopy.
*Coverage information as of September 2020
†DISCLAIMER: This is not a guarantee of payment, coverage, or reimbursement. Individual payer plans may vary.
Choose from 3 steroid-eluting implants under one brand to select the best option that conforms to the anatomical needs of your patients.
Visit the reimbursement resources for more information on processing and payment for PROPEL sinus implants when used in facility or non-facility settings.
VIEW REIMBURSEMENT RESOURCES
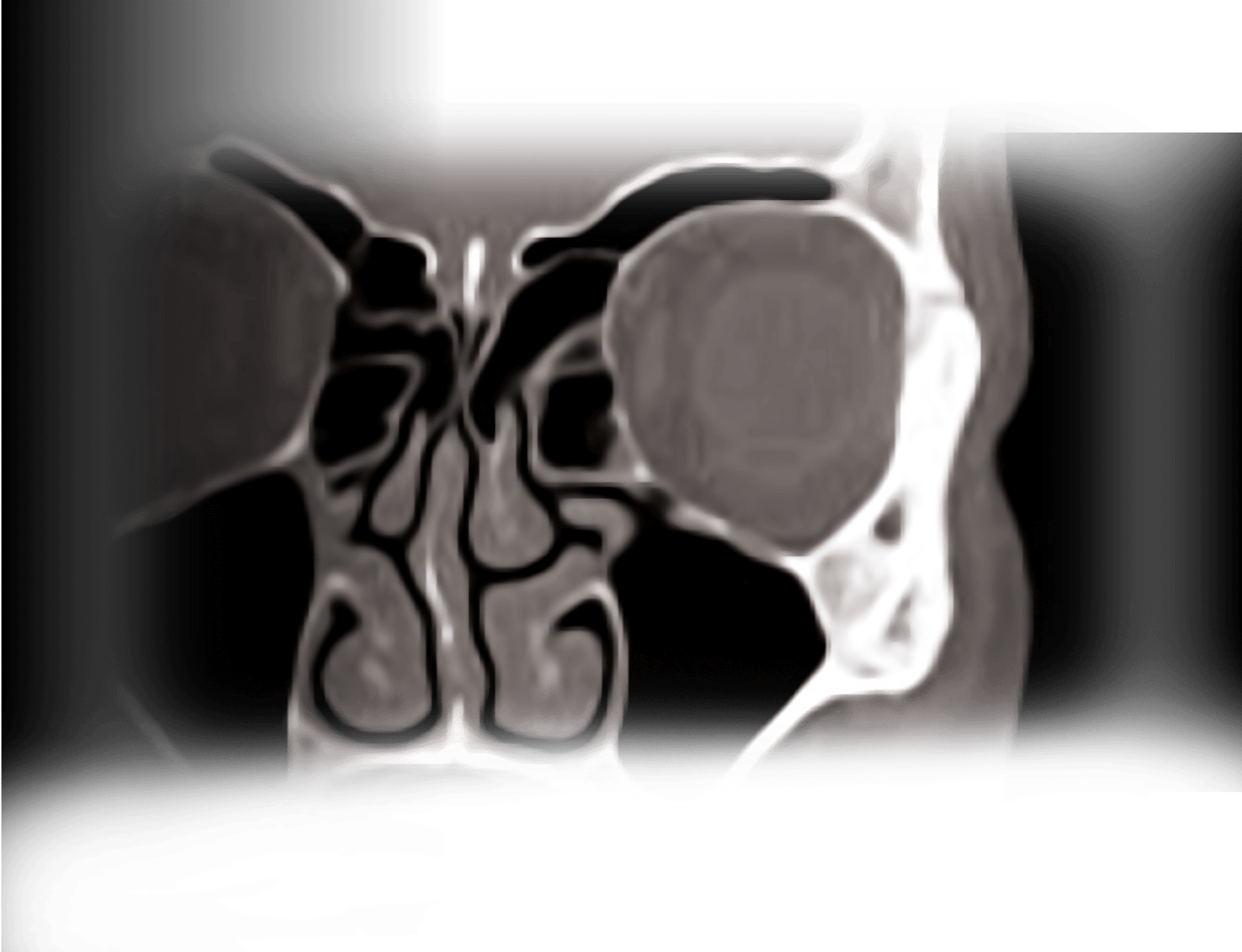
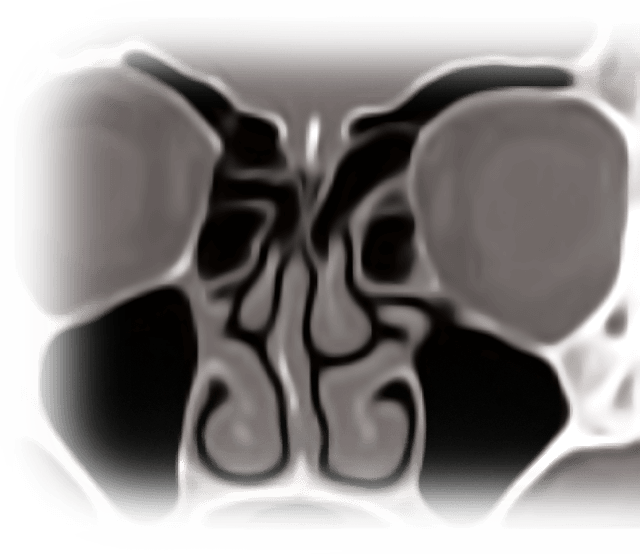



ETHMOID SINUS
PROPEL MINI (NDC: 10599-0001-01)
PROPEL (NDC: 10599-0000-01)
FRONTAL SINUS OSTIA
PROPEL MINI (NDC: 10599-0001-01)
PROPEL CONTOUR (NDC: 10599-0002-01)
MAXILLARY SINUS OSTIA
PROPEL CONTOUR (NDC: 10599-0002-01)
PROPEL implants were evaluated in clinical trials that included patients with and without nasal polyps, undergoing primary or revision surgery by traditional instrumentation or ballooning procedures5
GET PROPEL
Stay Informed
This site is intended for healthcare professionals in the United States