GET PROPEL
Stay Informed

The first real-world evidence analysis on the impact of the PROPEL family of implants in CRS patients
A retrospective, observational cohort study including 3,966 adult patients with CRS with or without nasal polyps that assessed the impact of the PROPEL family of implants on healthcare resource usage, in the 18 months following sinus surgery.
Real-World Evidence Study Design
A retrospective, observational cohort study*
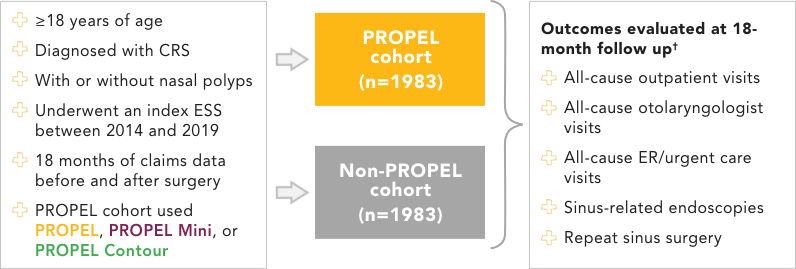
EMR, electronic medical record; HCRU, health care resource utilization.
*Leveraged a multisource database containing healthcare claims and EMR data in the US. The dataset used for this study is fully de-identified.
†Chi-square for binary variables and analysis of variance tests for continuous variables were applied to compare HCRU measures.
‡The logistic regression model included the following baseline variables: demographics (age, sex, race), presence of asthma and/or allergic rhinitis, overall comorbidity burden as measured by the Deyo-Charlson comorbidity index, year of surgery, duration of the baseline period, and facility type.
1. Hoffman V, et al. Curr Med Res Opin. 2022 Jan 20:1-7. doi: 10.1080/03007995.2021.2022874. Epub ahead of print.
PROPEL and non-PROPEL cohorts were well-balanced with nearly 10,700 total sinuses studied.
Real-World Evidence Patient Population
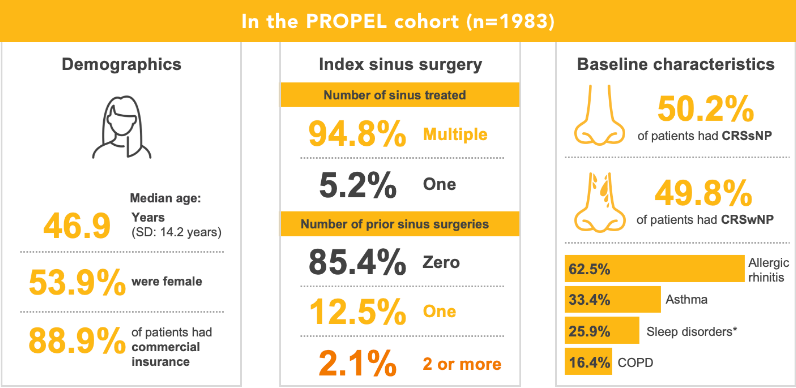
COPD, chronic obstructive pulmonary disease; SD, standard deviation.
*Includes sleep apnea, nighttime awakening due to congestion, and snoring.
1. Hoffman V, et al. Curr Med Res Opin. 2022 Jan 20:1-7. doi: 10.1080/03007995.2021.2022874. Epub ahead of print.
Real-World Evidence Study Limitation
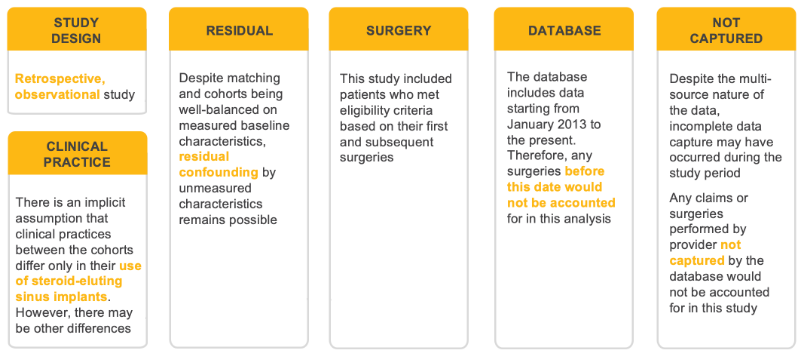
1. Hoffman V, et al. Curr Med Res Opin. 2022 Jan 20:1-7. doi: 10.1080/03007995.2021.2022874. Epub ahead of print.
23% relative reduction in all-cause otolaryngology visits1
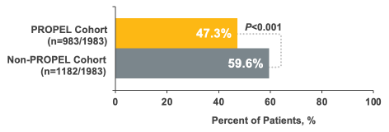
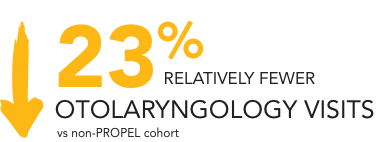
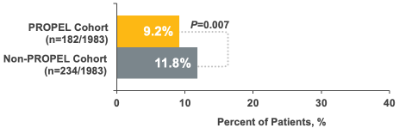
25% relative reduction in all-cause ER/urgent care visits1

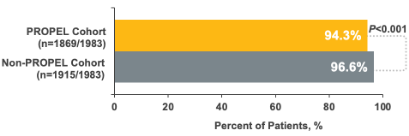
2% relative reduction in all-cause outpatient visits1

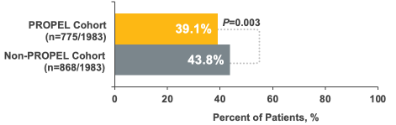
11% relative reduction in sinus-related endoscopies

14% relative reduction in repeat sinus surgeries1
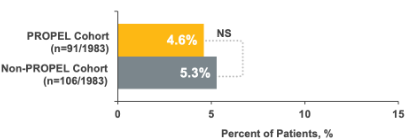

NS, not significant.
1. Hoffman V, et al. Curr Med Res Opin. 2022 Jan 20:1-7. doi: 10.1080/03007995.2021.2022874. Epub ahead of print
The use of PROPEL following sinus surgery may result in payer savings from reduced healthcare resource utilization
GET PROPEL
Stay Informed
This site is intended for healthcare professionals in the United States