GET PROPEL
Stay Informed

HOW DO YOU EVALUATE THE UNDERLYING INFLAMMATORY DISEASE IN PATIENTS WITH CRS? WATCH TO LEARN MORE

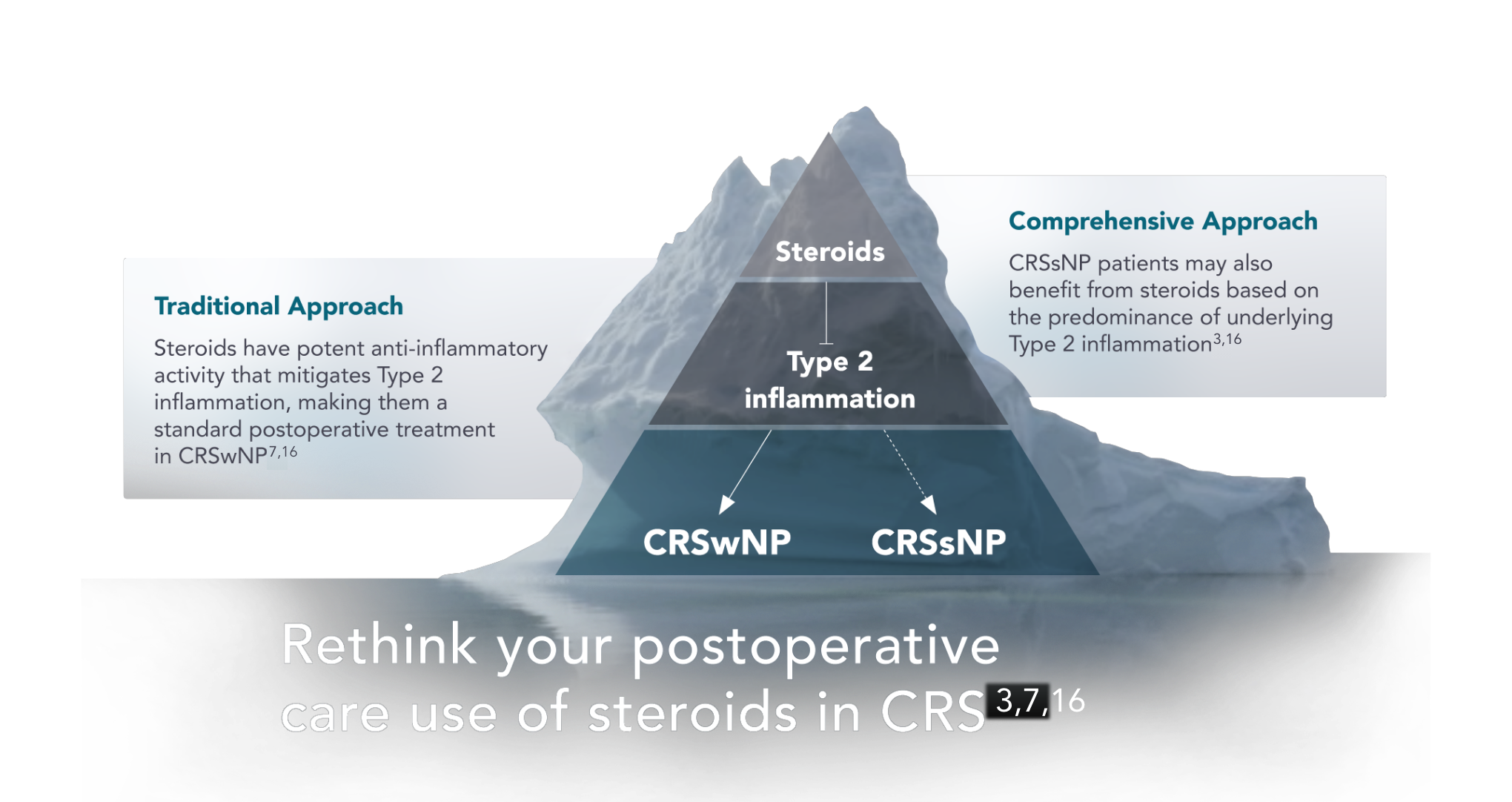
Chronic rhinosinusitis (CRS) is a heterogeneous disease, often characterized by the presence (CRSwNP) or absence (CRSsNP) of observable phenotypes like nasal polyps. However, phenotypes don’t necessarily reflect the underlying inflammatory mechanisms.1-3




CRS is an inflammatory disease, in which the underlying inflammation is largely characterized as either Type 1, 2, or 3.2,3,6
Chronic rhinosinusitis (CRS) is a heterogeneous disease, often characterized by the presence (CRSwNP) or absence (CRSsNP) of observable phenotypes like nasal polyps. However, phenotypes don’t necessarily reflect the underlying inflammatory mechanisms.1-3





FESS, functional endoscopic sinus surgery; IFN, interferon; IL, interleukin; OMC, osteomeatal complex.
*Type 2 inflammation only or a mixture of Type 2 with Type 1 and/or Type 3.
†Study evaluated the association between inflammatory endotypes and clinical presentations in CRS. Patients with CRSsNP (n=121) were compared with patients with CRSwNP (n=134) and markers including IFN-γ (Type 1), eosinophil cationic protein (Type 2), Charcot-Leyden crystal galectin (Type 2), and IL-17A (Type 3) were used to determine inflammatory endotypes.
‡As compared to 17% and 18% of CRSwNP patients have some degree of Type 1 and Type 3 inflammation, respectively.
§As compared to 21% and 27% of CRSsNP patients have some degree of Type 1 and Type 3 inflammation, respectively.

Surgery can help remove inflamed mucosal tissue; however, it does not address the root cause of the disease, and revision surgery is often needed.12 Alleviating persistent inflammation with intranasal steroids (INS) postoperatively is critical for achieving optimal surgical outcomes.13
of CRSwNP patients have recurrence of nasal polyps within 18 months postsurgery12
of CRSsNP patients require revision surgery in a 5-year follow-up14
Disease recurrence post surgery in CRSwNP patients without INS vs with INS, at 1 year15
Although oral corticosteroids (OCS) have an established role in postoperative care, and in many cases may be the right treatment for patients with CRS, it’s important to consider its challenges.1,17 Even short courses of OCS have been associated with systemic adverse reactions, especially when administered repeatedly.18
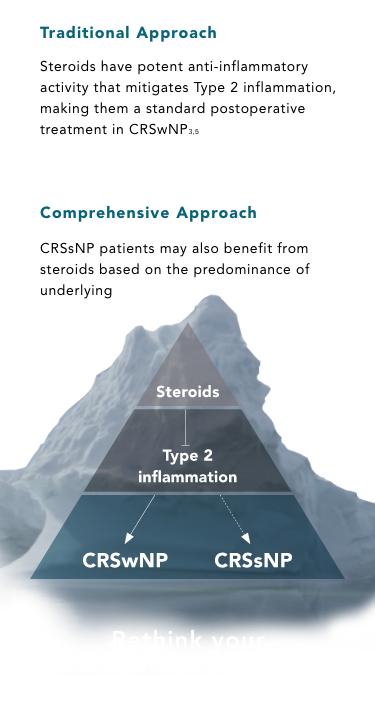
Not all steroids are created equal
Mometasone furoate (MF) is a next-generation corticosteroid designed for improved efficacy and safety.19
The PROPEL sinus implants are indicated to maintain patency and locally deliver steroid to the sinus mucosa in patients ≥18 years of age after sinus surgery: PROPEL for the ethmoid sinus, PROPEL Mini for the ethmoid sinus/frontal sinus opening, and PROPEL Contour for the frontal/maxillary sinus ostia.
Mometasone furoate has not been specifically studied on Type 2 inflammation, but PROPEL has been studied on both patients with CRSwNP and CRSsNP.







PROPEL implants feature an innovative 2-in-1 mechanism that opens the sinuses while delivering MF20-22*






*The precise mechanism behind the anti-inflammatory properties of the eluted MF is not known.20
†Postoperative interventions was a composite endpoint that included surgical intervention required to separate an adhesion and/or oral steroid intervention to resolve recurrent ethmoid sinus inflammation, edema, and/or polyp recurrence.23
‡Judged by an independent panel.23
Study design: Meta-analysis of two prospective, randomized, double-blinded multicenter studies (Pilot and ADVANCE II) that enrolled 143 patients.23
GET PROPEL
Stay Informed
This site is intended for healthcare professionals in the United States