GET PROPEL
Stay Informed









Hourglass-shaped
sinus opening


Cylindrical-shaped
sinus opening
≥18 years of age1,2
With and without polyps3,4
Undergoing primary or revision ethmoid sinus surgery3,4
Frontal sinus surgery by traditional instrumentation, balloon dilation, or a hybrid of both3,4


PROPEL Contour reduced the need for postoperative interventions vs surgery alone, at 30 days following frontal sinus surgery (11.5% vs 32.8%; N=80)*†

Significant improvements in occlusion/restenosis in patients who received PROPEL Contour vs Control through Day 90
*Postoperative interventions was a composite endpoint that included surgical intervention required to separate an adhesion and/or oral steroid intervention to resolve recurrent frontal sinus inflammation, edema, and/or polyp recurrence.
†Judged by an independent reviewer.
The benefits of PROPEL Contour in reducing the need for postoperative interventions vs surgery alone following frontal sinus surgery were supported by reductions in occlusion/
*Judged by on-site clinical investigators. Prespecified and adjusted for multiplicity.
Benefits were also observed in occlusion/restenosis and size of the frontal sinus ostia with PROPEL Contour vs surgery alone through Day 30, as per clinical investigators3
63% relative reduction in occlusion/restenosis
43% greater diameter of the frontal sinus ostia (FSO)
The FSO diameter of sinus implanted with PROPEL Contour contracted by 24%, as compared to 37% in surgery-only sinuses.
Study Design: The PROGRESS study was an 80-patient prospective, randomized, controlled, blinded clinical trial. The study evaluated outcomes of frontal sinus surgery (using balloons and/or traditional instruments) with PROPEL Contour placement compared to surgery alone, both with standard postoperative care. The study used an intra-patient control design to evaluate clinical outcomes. PROPEL Contour was removed at Day 21 to facilitate blinded independent assessment at Day 30.3

Learn more about the benefits of PROPEL Contour after frontal sinus surgery
For significant improvements to patient outcomes following sinus surgery, add PROPEL Contour to your battle for the frontal sinus
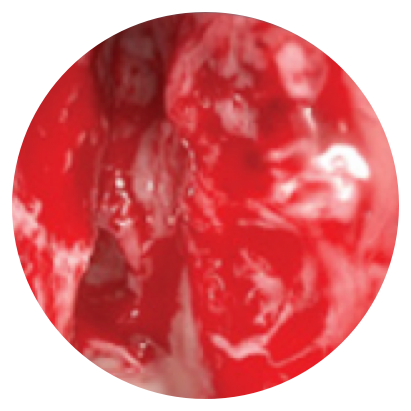
Day 0

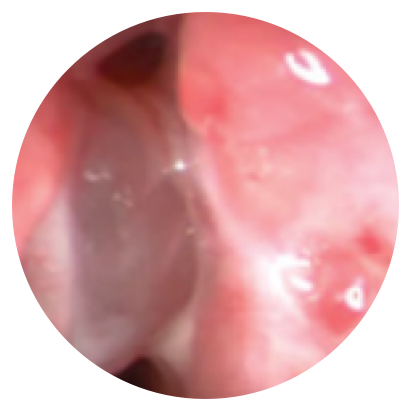
Day 30
Day 0


Day 30
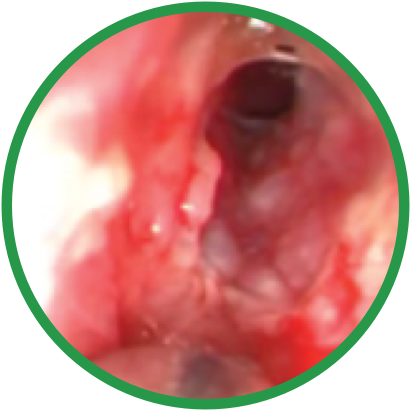
*Representative outcomes in bilateral sinuses of a single patient from a PROPEL clinical study. Individual results may vary.
The safety profile of PROPEL Contour was established in the PROGRESS study (Contour Cohort), which included 80 patients.1 The most common adverse reactions observed in ≥2% of subjects were acute sinusitis, asthma, headache, chronic sinusitis, upper respiratory tract infection, fungal sinusitis, nasopharyngitis, nausea, neck pain, sinus headache, and streptococcal pharyngitis.



PROPEL Mini reduced the need for postoperative interventions vs surgery alone, at 30 days following frontal sinus surgery (38.8% vs 62.7%; N=80)*†
*Postoperative interventions was a composite endpoint that included surgical intervention required to separate an adhesion and/or oral steroid intervention to resolve recurrent frontal sinus inflammation, edema, and/or polyp recurrence.
†Judged by an independent reviewer.
The benefits of PROPEL Mini in reducing the need for postoperative interventions vs surgery alone following frontal sinus surgery were supported by reductions in occlusion/
*Judged by on-site clinical investigators.
Benefits were also observed in inflammation and size of the frontal sinus opening, with PROPEL Mini vs surgery alone at Day 30, as per clinical investigators3
54% reduction in mean occlusion/restenosis
32% greater diameter of the frontal sinus opening5
Study Design:The PROGRESS study was an 80-patient prospective, randomized, controlled, blinded clinical trial. The study evaluated outcomes of frontal sinus surgery (using balloons and/or traditional instruments) with PROPEL Mini placement compared to surgery alone, both with standard postoperative care. The study used an intra-patient control design to evaluate clinical outcomes. PROPEL Mini was removed at Day 21 to facilitate blinded independent assessment at Day 30.4

Learn more about the benefits of PROPEL Mini after frontal sinus surgery
For significant improvements to patient outcomes following sinus surgery, add PROPEL Mini to your battle for the frontal sinus
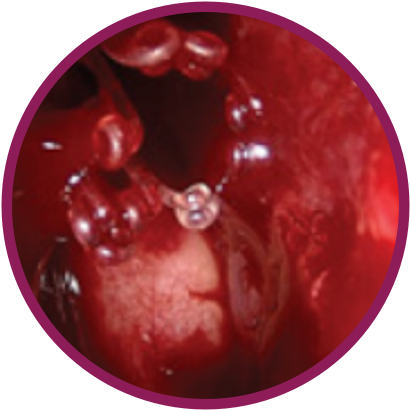
Day 0
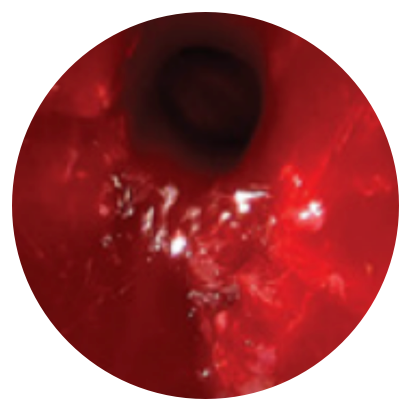

Day 30
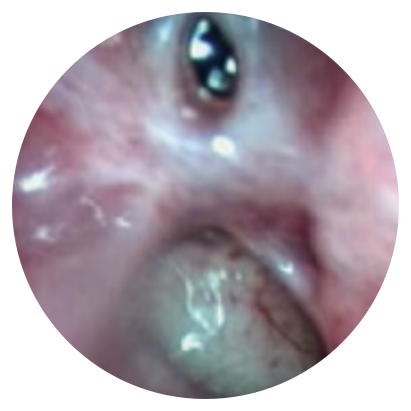
Day 0

Day 30
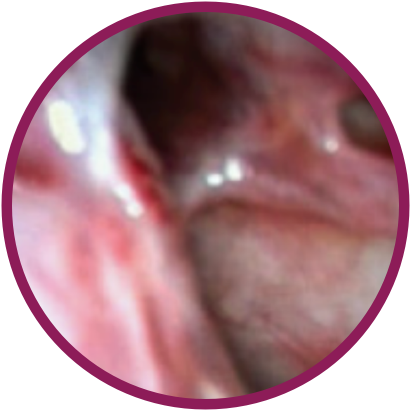
*Representative outcomes in bilateral sinuses of a single patient from a PROPEL clinical study.
Optimal treatment following frontal sinus surgery with a full arsenal of options including PROPEL Mini and PROPEL Contour, can help improve patient outcomes
The safety profile of PROPEL Mini was established in the PROGRESS study (Mini Cohort), which included 80 patients.2 The most common adverse reactions observed in ≥2% of subjects were acute sinusitis, chronic sinusitis, headache, upper respiratory tract infection, epistaxis, presyncope, acute otitis media, asthma, nasal congestion, eyelid edema, influenza, nasal polyps, nasopharyngitis, and nausea.
GET PROPEL
Stay Informed
This site is intended for healthcare professionals in the United States