GET PROPEL
Stay Informed



Watch Dr. McKinney discuss the case
Developed bacterial infection postsurgery
Infection was treated with antibiotics and saline rinses
CRS symptoms continued
Endoscopy reveals thick mucus and residual scar tissue
Next steps: revision maxillary
CRS, chronic rhinosinusitis.
*This case is intended for general medical education purposes only and is not a substitute for independent clinical medical judgment. Response to treatment may vary from patient to patient.

PROPEL Contour is an hourglass-shaped implant, 8 mm in nominal length, and indicated for the frontal and or maxillary sinus ostia1
PROPEL Contour delivery success rate (primary endpoint) in maxillary sinuses was 95.2% (N=15)
100% maxillary sinus ostial patency (secondary endpoint) was achieved at Day 30 (N=15)
Reduction in inflammation was observed at Day 30
Study Design: EXCEED was a 15-patient, prospective, single-arm, open-label feasibility trial. Patients were implanted with PROPEL Contour into the maxillary sinus ostia following sinus surgery with traditional instrumentation, balloon dilation, or a hybrid of both.1,3
For significant improvements to patient outcomes following sinus surgery, add PROPEL Contour to your battle for the maxillary sinus*
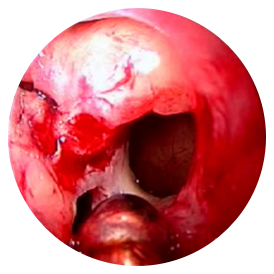
PRE-OP

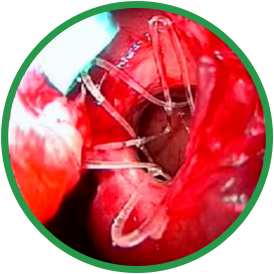
Post-ess

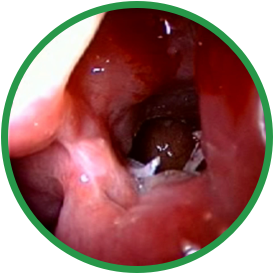
4 months post-ess
ESS, endoscopic sinus surgery.
*Representative outcome of a single patient. Individual results may vary.
Optimal treatment following maxillary sinus surgery with a full arsenal of options including PROPEL Contour can help improve patient outcomes
GET PROPEL
Stay Informed
This site is intended for healthcare professionals in the United States